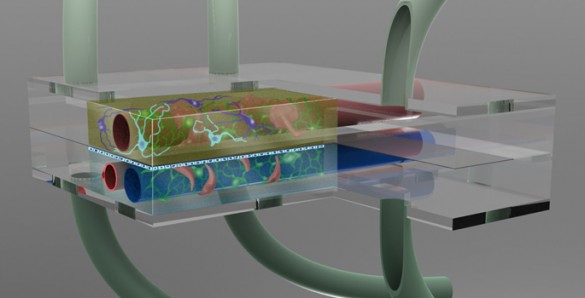
Take a millionth of a human brain and squeeze it into a special chamber the size of a mustard seed. Link it to a second chamber filled with cerebral spinal fluid and thread both of them with artificial blood vessels in order to create a microenvironment that makes the neurons and other brain cells behave as if they were in a living brain. Then surround the chambers with a battery of sensors that monitor how the cells respond when exposed to minute quantities of dietary toxins, disease organisms or new drugs under development.
Creating such a “microbrain bioreactor” is the challenge of a new $2.1 million research grant awarded to an interdisciplinary team of researchers from Vanderbilt University, Vanderbilt University Medical Center, the Cleveland Clinic and Meharry Medical College. The grant is one of 17 that are being issued by the National Center for Advancing Translational Sciences at the National Institutes of Health as part of a $70 million “Tissue Chip for Drug Testing” program. The five-year program is a cooperative effort on the part of NIH, the Defense Advanced Research Projects Agency and the FDA.
The reason for microfabricating organ simulators containing small populations of human cells – generally known as organ-on-a-chip technology – is to bridge the formidable gaps that exist between the tools that researchers currently use to develop new drugs – cell cultures and animal and human testing. These gaps not only add substantially to the difficulty and expense of developing new drugs but also contribute to the large number of experimental drugs that aren’t effective or have unacceptable side effects when they are finally tested on people.
The brain is a particularly difficult target for drug development because it is surrounded by three barriers that protect it from molecular or cellular intruders. The most formidable of these is the blood-brain barrier (BBB). It surrounds the blood vessels that service the brain and allows the passage of compounds that the brain needs while simultaneously blocking the passage of other types of molecules, both foreign and domestic. The two other barriers protect the neurons from contaminants in the cerebral spinal fluid and protect the cerebral spinal fluid from contaminants in the blood. Not only do these barriers block potentially harmful molecules, neuroscientists have also discovered that they occasionally alter the chemistry of some of the compounds that they let through.

“Given the differences in cellular biology in the brains of rodents and humans, development of a brain model that contains neurons and all three barriers between blood, brain and cerebral spinal fluid, using entirely human cells, will represent a fundamental advance in and of itself,” said John Wikswo, the Gordon A. Cain University Professor and director of the Vanderbilt Institute for Integrative Biosystems Research and Education (VIIBRE), who is orchestrating the multidisciplinary effort.
Wikswo and his collaborators argue that this new type of brain model should provide new insights into how the brain receives, modifies and is affected by drugs and disease agents. By replicating the forms of chemical communication and molecular trafficking that take place in the human brain, the device will allow them to test the effectiveness of various drug and nutritional therapies designed to prevent both acute injuries like strokes and chronic diseases like obesity and epilepsy, as well as uncovering the potential adverse effects of experimental drugs.
The basic microbrain bioreactor involves the integration of several technologies that have been developed and tested independently:
- Damir Janigro, director of the Cerebrovascular Research Center at the Cleveland Clinic (CC) and his coworkers have developed a hollow-fiber model of the BBB that uses two of the three cell types that make up the human barrier. Tests of this model have shown that it accurately reproduces a number of the features of the real BBB. Janigro’s team will adapt his model so that it will work with the new system, allowing for permeability and neurotoxicity studies of drugs. “There is huge need for predictive models of drug delivery to the brain. Neurological diseases are growing in our aging population and drug development for Alzheimer’s disease or other neurodegenerative diseases is of paramount importance for public health. The approach made possible by this multicenter grant will allow for a more rational drug design,” he said.
- Donald J. Alcendor, associate professor of microbiology and immunology at Meharry Medical College, will work with the CC and Vanderbilt teams to add the third cell type in the human BBB, called pericytes, to the microbrain bioreactor system, along with other cellular components that will allow the monitoring of molecular traffic for therapeutic analysis and cellular responses of the diseased brain. Alcendor discovered that Cytomegalovirus infection, which is the leading infectious cause of congenital disease in babies, spreads into the brain by infecting the pericytes. “The microbrain bioreactor should allow us to better understand how cell systems in the blood-brain barrier support and respond to Cytomegalovirus infection,” he said.
-

Donna Webb (Steve Green / Vanderbilt) A team headed by Donna Webb, assistant professor of biological sciences and Deyu Li, associate professor of mechanical engineering, will incorporate special microfluidic valves that they developed to study the interactions between neurons and the glial cells that accompany them in the brain.

Deyu Li (Daniel Dubois / Vanderbilt) These simple valves allow the scientists to maintain different types of cells in individual chambers linked by microchannels that can be easily opened and closed. This capability allowed Webb’s team to show that fluid from the glial cells is critical for neuron survival. “The new platform, with its unprecedented complexity, will greatly advance the lab-on-a-chip field,” Li said.
-

David Cliffel (Daniel Dubois / Vanderbilt) The VIIBRE group has extensive experience instrumenting extremely small bioreactors. Associate Professor of Chemistry David Cliffeland his group have been building miniature electrochemical sensors that measure cellular metabolism by tracking the consumption of glucose and oxygen and the production of lactate and changes in acidity.

John McLean (John Russell / Vanderbilt) Assistant Professor of Chemistry John McLean‘s group have been leading the effort to connect these small devices to sophisticated mass spectrometers that can identify in real time the presence of thousands of different proteins and other molecules that the cells produce. “It’s an exciting challenge to apply real-time analytics to decipher the molecular communication that takes place in the brain,” McLean said.

Once the microbrain bioreactor has been developed and tested, a team headed by Scott Daniels, assistant professor of pharmacology and director of drug metabolism and pharmacokinetics at the Vanderbilt Center for Neurosciences Drug Discovery, will collaborate with the Cleveland Clinic Foundation group to validate the chip technology by testing it with a number of clinically approved and experimental compounds that vary in their ability to penetrate the central nervous system. Daniels noted that “the brain-on-a-chip technology could enable the accurate prediction of human brain penetration by small molecule therapeutics and therefore bridge a critical translational gap in the drug discovery arena.”

Another group, headed by Associate Professor of Medicine Kevin Niswender, will be applying the new device to study the biology of stroke and the role that the brain plays in obesity. The system will allow his group to ask fundamental questions about how dietary macronutrients and inflammatory signals influence the various components present in the brain. Assistant Professor of Molecular Physiology and Biophysics Kate Ellacot will employ the system to understand how glial cells interact with neurons in the context of inflammation in obesity. In addition, the Cleveland Clinic has a large collection of samples from patients with these and other conditions. They will put cells from selected patients in the bioreactors and quantify how they respond to different treatments. “The ability to apply these precious samples entrusted to us by patients to a platform where we can literally measure hundreds of parameters is a dream come true,” said team member BethAnn McLaughlin, assistant professor of neurology and member of the Vanderbilt Kennedy Center. “We have enormous challenges in developing therapeutics to protect the brain from injury and this is a profound unmet need. Not a single drug has passed FDA approval to protect the brain from stroke, and we only have one that breaks up clots. We need to do better.”