By Alexandra Scammell
A group of Vanderbilt researchers has become the first group to uncover the 3D structure of the sodium/iodide symporter, also called NIS—the membrane protein that transports iodide into the thyroid to make thyroid hormones. This protein, which is critical for cell metabolism, is also integral to the most successful internal radiation cancer therapy: radioiodide treatment for thyroid cancer.
“What is really interesting about this protein is that it’s been used clinically, every day, for the diagnosis and treatment of thyroid disease since 1946,” said Nancy Carrasco, professor and chair of the Department of Molecular Physiology and Biophysics who launched the field of NIS research at the molecular level.

The study, “Structural insights into the mechanism of the sodium/iodide symporter,” was published in Nature on Dec. 14, 2022, and was spearheaded by Silvia Ravera. The work involved other members of the Carrasco laboratory and longtime collaborators from the Johns Hopkins University School of Medicine, the Yale School of Medicine and other renowned institutions.

Oxidized iodide, or iodine, is a key component of thyroid hormones. “Thyroid hormones are absolutely essential for the development and maturation of the central nervous system, skeletal muscle and lungs, and for intermediary metabolism in all cells,” Carrasco said.
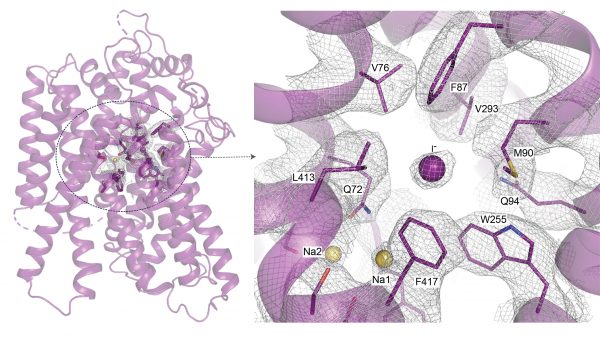
Transporting iodide into the thyroid can only be done by NIS. This capability is the most “striking attribute” of the protein, according to Carrasco. But how NIS selectively transports iodide in from outside the cell has been a long-standing question—one that can only be answered if the structure and transport mechanisms of NIS are better understood. Until this study, the structure of the protein had not been established.
To get a high-resolution view of the structure of NIS, the study authors first attempted to image it with X-ray crystallography, which can reveal the 3D organization of atoms within a crystalline solid. This plan failed, so they turned to cryo-electron microscopy, which flash-freezes a protein to showcase its structure. Using cryo-EM, the researchers collected millions of images of rat NIS, which has similar transport characteristics and sequence identity to those of human NIS, to determine the first 3D structure of the protein.
Acquiring and processing these images is a difficult task, Ravera said. One particularly challenging aspect was that NIS is completely embedded in the cell membrane and does not have any distinguishable features to help the researchers analyze the images and create a 3D reconstruction. “Our protein was very difficult because we didn’t have any striking features in the protein’s structure to help this process,” Ravera said.
Erkan Karakas, an assistant professor in the Department of Molecular Physiology and Biophysics, was instrumental in advising the team on how to process the data and analyze the images.

Once the researchers revealed the structure of NIS, they worked to determine how NIS binds iodide. “Understanding how NIS binds iodide … this could eventually be very useful therapeutically,” Carrasco said. For instance, NIS also transports the environmental pollutant perchlorate into thyroid cells, and high concentrations of perchlorate can lead to an underactive thyroid, or hypothyroidism.
Ravera added that “the more we know about the protein, the more that can be developed” therapeutically.
The findings of this landmark study have implications for treating patients with thyroid and other types of cancers. The scientists believe these findings can provide a foundation for research looking to extend radioiodide treatment to other malignancies, such as breast cancer. In addition, this new work can provide experts with a structural, mechanistic understanding of how NIS transports other substrates and how mutations in NIS cause iodide deficiency disorders—which, unless detected and treated shortly after birth, lead to cognitive deficits.
According to Carrasco and Ravera, this work could not have been done without the collaboration of their coauthors. “This protein has taken us to fields that we did not anticipate—from public health to breast cancer” and beyond, Carrasco said.
Carrasco, who has been studying this protein for decades, and other scientists will continue to investigate this protein in the future. “We think that now, this is just the tip of the iceberg,” she said, adding that the “second episode” of this story will be engineering molecules that could be used to treat a wide variety of diseases beyond thyroid cancer.