THE IDEA
A team of researchers led by Oak Ridge National Laboratory microscopist Miaofang Chi and Vanderbilt theoretical physicist Sokrates Pantelides has used a new Scanning Transmission Electron Microscope technique to image the electron distribution in ionic compounds known as electrides— especially the electrons that float loosely within pockets and appear separate from the atomic network.


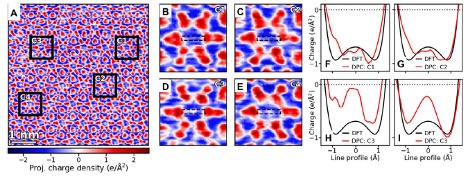
The new technique, Differential Phase Contrast in STEM, measures and maps electric fields and charge distributions inside a material. The study is the first time that DPC has been used in this way. By analyzing charge images of dozens of such channels, the team found that only some contain the negative charge predicted by theoretical calculations, while others have significantly less negative or even a small concentration of positive charge. Pantelides’ decades of experience with hydrogen led to the suggestion that traces of hydrogen, which are essentially impossible to eliminate, are responsible for the observed inhomogeneity, and subsequent detailed calculations confirmed the hypothesis. Neutron scattering experiments provided evidence in support of the hydrogen scenario.
WHY IT MATTERS
Pantelides expects that many physicists and engineers will be using the results of this study to inform their research, as all modern technology is built on electronic properties of materials.
A frontier research area that took off in the last 10 years, “electrides were slow to understand because of their strange properties,” said Chi, a research staff member at the Center for Nanophase Materials Sciences at ORNL. “This work provides a technique that directly visualizes and quantifies these electrons that behave like an atom with no nucleus, providing a unique tool to investigate electrides.”
“The materials are promising,” said Pantelides, University Distinguished Professor of Physics and Engineering and William A. & Nancy F. McMinn Professor of Physics. “We anticipate that this work will be used in both experimental and theoretical analysis of the exotic properties in electrides and the role that hydrogen may have in their behavior.”
WHAT’S NEXT
Currently, computer scientists are deploying machine-learning techniques to quickly identify materials with electride signatures so they can be further investigated. It is already known that electrides are good for storing hydrogen, can be used as catalysts, carry strong currents because of their high electron mobility and often exhibit unconventional magnetism, even superconductivity. These and other properties make their development attractive for an array of emerging technologies.
FUNDING
This research was funded by a Department of Energy Office of Science Early Career project FWP# ERKCZ55 – KC040304, the Department of Energy Office of Science, Basic Energy Sciences, Materials Science and Engineering grant DE-FG02-09ER4654, the McMinn Endowment at Vanderbilt, the Japanese Ministry of Education, Culture, Sports, Science and Technology and the Japan Science and Technology Agency.
GO DEEPER
Researchers on the project included Tianli Feng, a recent postdoctoral researcher in Pantelides’ research group, Qiang Zheng, a postdoctoral researcher at ORNL, and Jordan Hachtel, a microscopist staff scientist at ORNL. Other scientists from ORNL, the University of Tennessee–Knoxville and the University of Tokyo participated in the work.
The article, “Direct visualization of anionic electrons in an electride reveals inhomogeneities,” by Qiang Zheng, Tianli Feng, Jordan A. Hachtel, Ryo Ishikawa, Yongqiang Cheng, Luke Daemen, Jie Xing, Juan Carlos Idrobo, Jiaqiang Yan, Naoya Shibata, Yuichi Ikuhara, Brian C. Sales, Sokrates T. Pantelides and Miaofang Chi was published in the journal Science Advances on April 7.