A team of scientists at Vanderbilt has discovered the first known instance of a hybrid fungal species causing aspergillosis, an ensemble of different types of lung infections that often impacts immune-compromised people.
Aspergillosis has long been causally connected to Aspergillus fungi, a group of more than 300 species of filamentous fungi that includes the fungus that produces penicillin. However, this new discovery will set the stage for future research on innovative identification, treatment, and management strategies for these lung diseases.
The findings by researchers in the laboratories of Antonis Rokas, Cornelius Vanderbilt Chair in Biological Sciences and director of the Vanderbilt Evolutionary Studies Initiative, and Gustavo Goldman, professor at São Paulo University and a faculty affiliate at Vanderbilt’s Center for Latin American Studies, were published online on June 4 in the journal Current Biology.
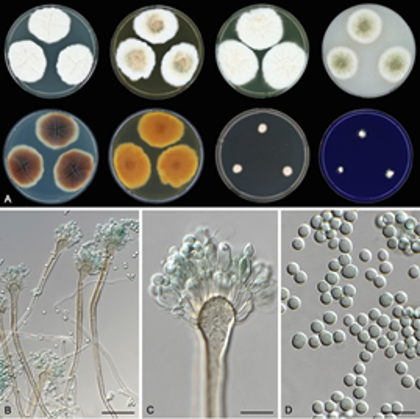
Led by graduate student Jacob L. Steenwyk and postdoctoral fellow at Gladstone Institutes and former graduate student Abigail L. Lind, the discovery shows proof of the hybrid species, Aspergillus latus infecting humans and is the first example of an Aspergillus hybrid in the clinical environment.

The global team of collaborators from the University of São Paulo in Brazil, the University of Minho in Portugal, as well as from the Catholic University of Leuven and the University Hospitals in Leuven in Belgium collected fungal isolates from patients infected with Aspergillus fungi for nearly a decade to figure out what makes these organisms pathogenic. Clinical microbiology labs, which typically identify infectious fungi using microscopy, thought all isolates belonged to Aspergillus nidulans. However, the Vanderbilt team discovered that some isolates were instead A. latus, a previously little-noticed fungus.
“This is one of the most surprising discoveries in my entire career,” Rokas said. “Gustavo sent us the DNA of isolates that different clinical labs thought belonged to A. nidulans. When we sequenced them, we quickly found that they were very different from A. nidulans.”
Through detailed evolutionary and genetic investigations Steenwyk, Lind, and their collaborators realized that A. latus originated from the hybridization of two species. “We think A. latus originated from two fungi fusing,” said Steenwyk. “Fusion, which is completely different from how animal hybrids come to be, has a unique biological outcome.”
Take the mule, for example. Hybrids familiar to us inherit half of their genome from one parent (in this case, a horse) and half from the other (a donkey). A. latus, however, did not inherit half of each parental genome but the whole of both parental species. “The genomes looked like they have two of everything; twice the genome size, twice the number of genes, and so on,” Lind says.
Using additional experiments, the research team was able to identify both parental species to determine what parts of the genome came from which parent. Comparisons of these genomes revealed the parental species were substantially different from one another. “We determined each parent is approximately as distinct as a human is to a lemur, which highlights the flexibility in how some fungi can evolve,” added Steenwyk.
A common observation in hybrid organisms is that they display characteristics of both parental species; for example, the mule has both donkey traits, such as intelligence and patience and horse traits, such as physical strength and speed. To test whether this was the case for A. latus, Steenwyk, Lind, and colleagues compared how well the fungus grows relative to its parents in diverse infection-relevant conditions, including in the presence of drugs used to treat fungal infections. Much like the mule, the researchers found that hybrid fungus had traits from both parents.

To combat infections, understanding how fungi respond to antifungal drugs is essential. “Examining how the fungus grows in infection-relevant conditions is the first step to helping patients impacted by these types of infections,” said Goldman.
The research is funded by the National Science Foundation, the Vanderbilt Discovery Grant Program, the Guggenheim Foundation, the Howard Hughes Medical Institute through the James H. Gilliam Fellowships for Advanced Study program, and a U.S. National Library of Medicine training grant. International funding was provided by the Brazilian São Paulo Research Foundation (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) as well as the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER).