Buddy Creech
-
Vanderbilt awarded up to $46M through ARPA-H to develop tools against alphaviruses
Jens Meiler, Distinguished Research Professor of Chemistry, was named the principal investigator for the project, in cooperation with 14 investigators across eight institutions. The team will work together to use advanced technology, including computational modeling, AI-driven predictions and structural biology, to develop a groundbreaking vaccine against all alphaviruses that offers long-lasting protection. Read MoreDec 2, 2024
-
Study finds Moderna’s COVID-19 vaccine safe and effective for young children
A Vanderbilt study finds that Moderna’s COVID-19 vaccine is safe and effective in children 6 months to 5 years of age. Read MoreNov 4, 2022
-

VUMC-led study finds Moderna COVID vaccine safe and effective for children
Moderna’s COVID-19 vaccine is safe and generates robust immune responses in children ages 6 to 11 years, a national clinical trial co-led by C. Buddy Creech, MD, MPH, has found. Read MoreMay 12, 2022
-

36 scholars honored at endowed chair investiture ceremony
Vanderbilt Chancellor Daniel Diermeier and Provost and Vice Chancellor for Academic Affairs C. Cybele Raver honored 36 scholars from across the university at an endowed chair investiture ceremony on campus March 30. Read MoreMar 31, 2022
-
VUMC begins study of second COVID-19 vaccine
Vanderbilt University Medical Center has begun recruiting up to 250 participants for a Phase 3 clinical trial testing an investigational COVID-19 vaccine candidate developed by the Janssen Pharmaceutical Companies of Johnson & Johnson. Read MoreNov 2, 2020
-
Remdesivir helps reduce COVID-19 recovery time: study
The investigational antiviral drug remdesivir can shorten the time to recovery in adults hospitalized with COVID-19, according to preliminary results of a clinical trial published last month in The New England Journal of Medicine. Read MoreJun 4, 2020
-

Buddy Creech, BS’95, MPH’06: Leading COVID-19 treatment trials
Buddy Creech, director, Vanderbilt Vaccine Research Program, is helping lead some of the NIH-funded treatment trials of COVID-19 in hospitalized patients at Vanderbilt University Medical Center. Read MoreMay 15, 2020
-
Team seeks to identify immune response to influenza
Vanderbilt researchers led by Buddy Creech are searching for the key to lasting protection against influenza by examining naturally protecting cells found in bone marrow. Read MoreNov 1, 2018
-

Volunteers sought for bird flu vaccine trial
Vanderbilt University Medical Center is recruiting volunteers to participate in a national study of an investigational vaccine against the H7N9 influenza virus, also known as “bird flu.” Read MoreMar 15, 2018
-
Vanderbilt leads international effort to develop universal flu vaccine
Researchers at Vanderbilt University Medical Center are leading an international effort to develop a universal influenza vaccine that would protect everyone against all strains of the flu anywhere in the world. Read MoreOct 26, 2017
-
New approach for staph-related skin abscesses explored
New multicenter research that includes Vanderbilt University Medical Center (VUMC) investigators, could change treatment approaches to simple skin abscesses, infections often caused by Staphylococcus aureus (staph) bacteria. Read MoreJul 13, 2017
-
Study tests shorter antibiotic course in children
Researchers at Vanderbilt University Medical Center (VUMC) are leading a multicenter clinical trial to evaluate whether a shorter course of antibiotics — five days instead of 10 — is effective at treating community-acquired pneumonia (CAP) in children who show improvement after the first few days of taking antibiotics. Read MoreDec 1, 2016
-

NIH launches website for StoryCorps project
The National Institutes of Health (NIH) is launching its Voices of the NIH Community website, which features a collection of StoryCorps audio recordings from patients, families, researchers, doctors, nurses, staff and volunteers in both the NIH and Vanderbilt University Medical Center (VUMC) communities. Read MoreJul 28, 2016
-

Creech to direct Vanderbilt Vaccine Research Program
Buddy Creech, M.D., MPH, associate professor of Pediatrics, has been named director of the Vanderbilt Vaccine Research Program (VVRP) in the Division of Pediatric Infectious Diseases. Read MoreOct 8, 2015
-
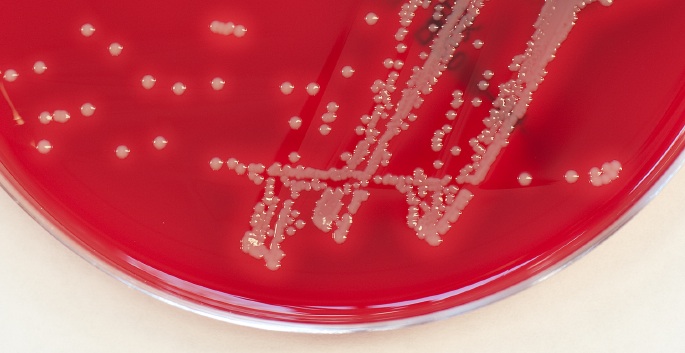
Staph ‘gangs’ share nutrients during infection: study
Antibiotic-resistant bacteria can share resources to cause chronic infections, Vanderbilt investigators have discovered. The findings shed light on a long-standing question in infectious diseases and may inform new treatment strategies. Read MoreOct 16, 2014
-

Study finds college athletes more likely to harbor MRSA
College athletes who play contact sports are more than twice as likely to carry the deadly superbug methicillin-resistant Staphylocuccus aureus (MRSA) than peers who play non-contact sports, according to a Vanderbilt study released at IDWeek 2014. Read MoreOct 9, 2014
-

Vaccine Research Program lands major NIH renewal
The Vanderbilt Vaccine Research Program has received a major contract from the National Institutes of Health to continue its work as one of the nation’s Vaccine and Treatment Evaluation Units. Read MoreSep 26, 2013
-
CDC selects VU to lead national adverse vaccine event reviews
Vanderbilt University Medical Center has been selected by the Centers for Disease Control and Prevention (CDC), to lead a consortium of top national experts in vaccine safety in performing timely reviews of adverse vaccine events. Read MoreDec 13, 2012
-

MRSA in pregnancy may be less dangerous than previously thought
Vanderbilt pediatric infectious disease researchers studying antibiotic-resistant staph say fears that mothers carrying the germ may set their newborns up for infection are unfounded. Read MoreApr 19, 2012
-

VUCast: Close to Reality?
This Week on VUCast: Why a Vanderbilt disease expert says the medical thriller “Contagion” may be closer to reality than you think [ watch full video of Dr. Creech and more information about vaccines ] Why it’s worth going to… Read MoreSep 30, 2011