A new cellular biology study, published last month in the journal Structure by scientists at Vanderbilt, reports a shape-shifting structure in the human body which plays an important role in the timely delivery of fats and proteins.
Led by Lauren Jackson, assistant professor of biological sciences and biochemistry at Vanderbilt, the work is the first to visualize this structure—a type of protein complex found in human cells known as retromer—and report its unique ability to transfigure itself into a variety of different architectures and structures.
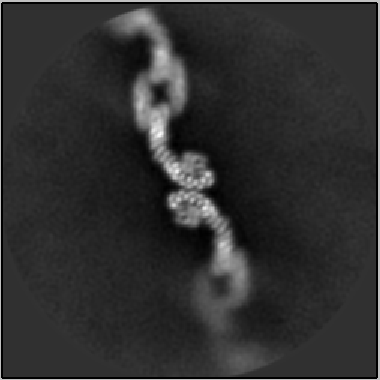
“Our work marks the first direct visualization of mammalian retromer,” said Jackson. “While structures of the protein have been determined from yeast, we can now begin to understand the more complex and intricate adjustments and movements happening in mammals to ensure our bodies function correctly.”

The human body relies on this parcel delivery process (referred to by Jackson as a “biological FedEx system”) to deliver important protein and fatty lipid molecules to the right places at the right times. In the event of delivery failures or interruptions, cells lose what they need to function and human diseases and neurological disorders such as Alzheimer’s and Parkinson’s can emerge.
To study the delivery process in the body and confirm the existence of retromer in cellular sorting stations called endosomes, the research team leveraged a cutting-edge structural biology method known as single particle cryo-electron microscopy, which uses beams of electrons at the atomic scale to project an image of a sample back onto a detector.
By visualizing retromer at the atomic level, the researchers captured (as seen in the image above) photographic evidence for one of the structure’s abilities: arranging itself in a scaffolding pattern—which specifically allows the sorting, re-direction and delivery of important proteins and lipids to a set of new destinations.
“This flexible scaffold structure plays a key role in the sorting and delivery process,” said Jackson. “These structures reveal how one complex alone is able to sort and deliver cellular ‘cargo’ to different destinations.”
The work also has important implications for gaining a greater understanding of disease and treatment pathways. Future studies will investigate mutations that interrupt deliveries, as well as identification of small molecules that may stabilize key scaffold structures
Electron microscopy data collection was performed at the Simons Electron Microscopy Center and National Resource for Automated Molecular Microscopy located at the New York Structural Biology Center, which is supported by grants from the Simons Foundation and the National Institutes of Health, National Institute of General Medical Sciences. Additional research is supported by grants from the National Institutes of Health and Pew Charitable Trusts.