
The year has gotten off to a good start for modern-day alchemists like Vanderbilt physicists Joe Hamilton and A.V. Ramayya who are engaged in the extremely challenging scientific endeavor of extending the periodic table by creating new elements.
In November, the International Union of Pure and Applied Chemistry (IUPAC) officially added three new superheavy elements – 110, 111 and 112 – to the periodic table with the names of darmstadtium (Ds), roentgenium (Rg) and copernicium (Cn). Then, just before Christmas, it proposed official names for two more additions: flerovium (Fl) for element 114 and livermorium (Lv) for element 116. The two new elements must now undergo a five-month public comment period before they are officially added.
In recent years, nuclear physicists have been creating new superheavy elements at a rate of about one every other year. The net result is a modern periodic table

that extends continuously from hydrogen, element one, to element 118, which was created in 2002. Nuclear physicists have reported the discovery of the last four of these elements – 113, 115, 117 and 118 – but IUPAC does not consider the evidence conclusive so it has given them temporary names and requested additional evidence.
In January, significant progress was made toward filling three of these remaining placeholders. An article published in the Physical Review Letters by a Russian-American scientific team including Hamilton, who is the Landon C. Garland Distinguished Professor of Physics, and Professor of Physics Ramayya reported the results of a series of experiments that produced significant quantities of the elements 113 and 115: definitively confirming the initial discovery in 2004. The experiments, conducted with scientists from the Russian Joint Institute for Nuclear Research, Lawrence Livermore National Laboratory and Oak Ridge National Laboratory also provided additional evidence supporting the discovery of element 117 in 2010.
These painstaking campaigns require months to years to conduct and, when successful, typically yield only a few nuclei of the new superheavy element. To create a new element, the scientists use powerful particle accelerators to slam beams with millions of heavy ions into targets made from exotic radioactive materials. When they are successful, these subatomic bombardments cause a few of the ions to fuse with the target nuclei to produce some nuclei of the new element, which typically exist for a brief period before decaying. This gives the scientists a very short window to identify the new nuclei and measure their physical properties. (The Joint Institute for Nuclear Research in Russia has a cyclotron specifically designed for this purpose and has created many of the newest superheavy elements.)
There is much more to the search for new elements than the brute force of atom smashing. Selecting the atoms to use in the beam and target is a major scientific challenge. Atomic nuclei contain two basic particles: protons and neutrons. Atoms with the same number of protons but different numbers of neutrons are called isotopes: They are chemically identical but have slightly different weights. The proportion of protons and neutrons plays a critical role in determining an isotope’s stability. Scientists have discovered that nuclei with even numbers of protons and neutrons are more stable than those with odd numbers. In addition, there are specific “magic numbers” of protons and neutrons that are especially stable. The magic numbers are 2, 8, 20, 28, 50, 82 and 126. “Double-magic” nuclei with magic numbers of both protons and neutrons are the most stable of all.
To successfully create a new element, the numbers of protons and neutrons in the target and beam atoms must add up correctly so they will fuse together to create a longer-lived isotope of the new element. For example, element 117 was created by fusing berkelium (element 97) with calcium (element 20). But the primary isotope of berkelium can only be made in a special research reactor and only a bit more than a gram has been produced in the U.S. since 1967. In addition, berkelium is radioactive and has a half-life of 330 days so it must be used promptly after it is created. One of the advantages of using calcium is that it has six stable isotopes and the heaviest, calcium-48, is double magic. This allowed the scientists to use calcium-48 with its 28 neutrons so that it would fuse with the isotope of berkelium, which has 152 neutrons to produce two of the longer-lived isotopes of element 117 which have 176 or 177 neutrons.
There are two basic reasons why scientists like Hamilton and Ramayya pursue these difficult scientific quests.
The first is that these experiments provide extremely sensitive tests of the accuracy of the current understanding of nuclear physics and chemistry. In particular, these discoveries are helping scientists understand how nuclei hold together and resist the fission process. This understanding helps maintain the viability of the nation’s nuclear arsenal and enhance the security of its nuclear reactors.
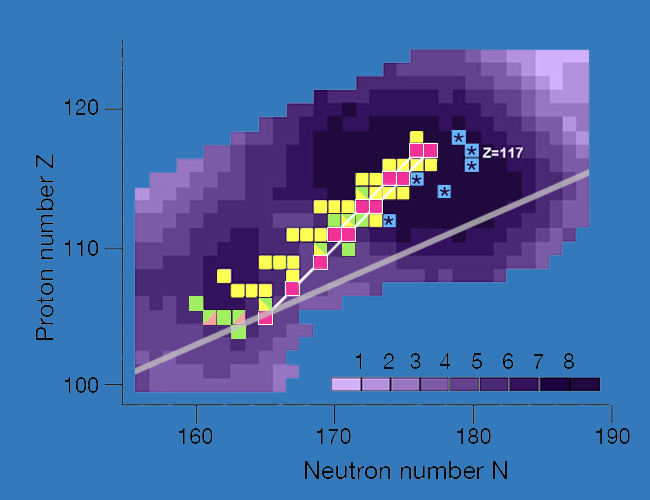
The second reason is that nuclear theory predicts that a superheavy “island of stability” should lie between element 114 and 126. According to this prediction, superheavy elements in this region that contain 184 neutrons could have lifetimes that are 100 to 1,000 times longer than most other artificial isotopes, many of which only exist for milliseconds or nanoseconds before decaying. The properties of the newest elements 113, 115 and 117 strongly support this prediction. In fact, the isotope of element 117 with 177 neutrons is the new isotope that is closest to the island of stability that the physicists have managed to create.
Scientists do not yet know of any practical applications for these new elements, but previously discovered artificial elements have found valuable applications: americium is used in smoke detectors; curium and californium are used in medical neutron radiography and plutonium is used in nuclear weapons and electrical generators for space probes.