At the same time Honda and Toyota are introducing fuel cell cars to the U.S. market, a team of researchers from Vanderbilt University, Nissan North America and Georgia Institute of Technology have teamed up to create a new technology designed to give fuel cells more oomph.
The project is part of a $13 million Department of Energy program to advance fuel cell performance and durability and hydrogen storage technologies announced last month.

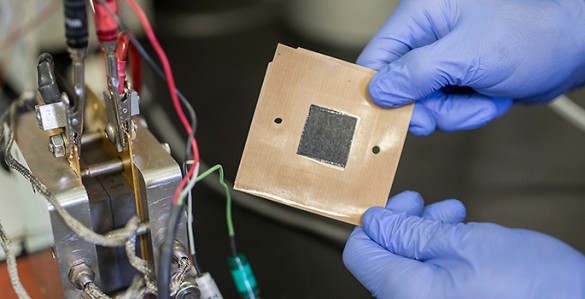
The $2.5 million collaboration is based on a new nanofiber mat technology developed by Peter Pintauro, the H. Eugene McBrayer Professor of Chemical Engineering at Vanderbilt, that replaces the conventional electrodes used in fuel cells. The nanofiber electrodes boost the power output of fuel cells by 30 percent while being less expensive and more durable than conventional catalyst layers. The technology has been patented by Vanderbilt and licensed to Merck KGaA in Germany, which is working with major auto manufacturers in applying it to the next generation of automotive fuel cells.
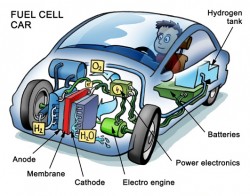
Fuel cells were invented back in 1839 but their first real world application wasn’t until the 1960’s when NASA used them to power the Apollo spacecraft. Fuel cells need fuel and air to run, like a gasoline engine, but they produce electricity, like a battery. In hydrogen/air fuel cells, hydrogen flows into one side of the device. Air is pumped into the other side. At the anode, the hydrogen is oxidized into protons. The protons flow to the cathode where the air is channeled, reducing the oxygen to form water. Special catalysts in the anode and cathode allow these reactions to occur spontaneously, producing electricity in the process. Fuel cells convert fuel to electricity with efficiencies ranging from 40 percent to 60 percent. They have no moving parts so they are very quiet. With the only waste product being water, they are environmentally friendly.
Porous polymer fiber mats replace solid electrodes
Conventional fuel cells use thin sheets of catalyst particles mixed with a polymer binder for the electrodes. The catalyst is typically platinum on carbon powder. The Vanderbilt approach replaces these solid sheets with mats made from a tangle of polymer fibers that are each a fraction of the thickness of a human hair made by a process called electrospinning. Particles of catalyst are bonded to the fibers. The very small diameter of the fibers means that there is a larger surface area of catalyst available for hydrogen and oxygen gas reactions during fuel cell operation. The pores between fibers in the mat electrode also facilitate the removal of the waste water. The unique fiber electrode structure results in higher fuel cell power, with less expensive platinum.
Octagonal catalyst particles more effective
Meanwhile at Georgia Tech, biomedical engineering professor Yuounan Xia has developed a method for carefully controlling the shape of nanoparticle catalyst for fuel cells. In particular, he has produced platinum-nickel nanoparticles with a regular octagonal shape. Theoretically, these particles should be more effective than commercial platinum black powder now used as the oxygen reduction catalyst in hydrogen/air fuel cells.
“The combination of the Georgia Tech catalyst with Vanderbilt’s nanofiber electrode technology could be a game-changer for the development and commercialization of automotive fuel cells,” Pintauro said.
In the new DOE project, Pintauro’s group will make nanofiber mat electrodes containing Xia’s nanoparticle catalysts. The electrodes will then be sent to Nissan Technical Center North America where a team of researchers led by Nilesh Dale, the manager of fuel cell research, will evaluate their performance under automotive operating conditions.
The research teams will also be working with scientists at the national labs – Oak Ridge National Laboratory, Los Alamos National Laboratory, the National Renewable Energy Laboratory, and Lawrence Berkeley National Laboratory – in an effort to improve the scientific understanding of why fuel cells work better with nanofiber mat electrodes.
“Right now, much of the research work on fuel cell electrodes is very Edisonian,” said Pintauro. “It is mainly trial and error experiments. We don’t know what will happen when we change the composition or structure of the electrodes in hydrogen/air fuel cells. With a better understanding of the interdependence of composition and nanostructure for fiber electrodes, we could accelerate the pace of our research, which would help us to achieve the cost and performance targets needed for automotive fuel cell commercialization.”