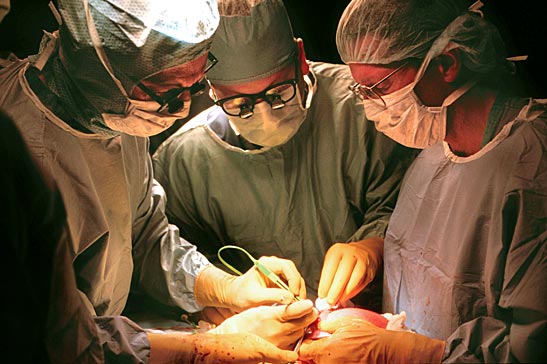
Results of a landmark, seven-year National Institutes of Health-funded trial, Management of Myelomeningocele Study (MOMS), demonstrate clear benefit for babies who undergo fetal surgery to treat spina bifida, the most common birth defect in the central nervous system.
The surgical procedure, in utero repair of myelomeningocele, was pioneered at Vanderbilt University Medical Center in 1997, with the first procedure performed on Corey Meyer of Mt. Juliet, Tenn., and her unborn son Daniel.
Enrollment in the MOMS trial was halted in December 2010, because researchers at the study’s three
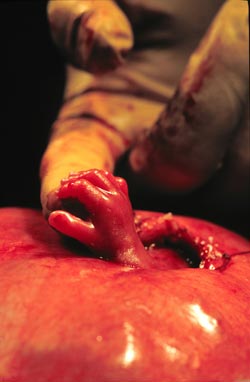
trial sites– Vanderbilt, the University of California San Francisco and Children’s Hospital of Philadelphia –found the procedure demonstrates significant benefit over the current standard of care, surgical repair after birth.
The findings, published in the Feb. 9 online issue of the New England Journal of Medicine, show babies who have corrective surgery for a serious form of spina bifida (myelomeningocele) while still in the uterus, experience a reduction in potentially life-threatening hydrocephalus and have
an increased ability to walk.
“The results are very exciting and confirm what our surgeons and researchers found back in 1999,”
said John Brock III, surgeon-in-chief for the Monroe Carell Jr. Children’s Hospital at Vanderbilt, and
principal investigator for the Vanderbilt site of MOMS.
Brock said teams of Vanderbilt experts, led by maternal/fetal surgeon Joseph Bruner, who is no
longer with Vanderbilt, and Noel Tulipan, director of pediatric neurosurgery, pursued research in the early 1990s that led to the first such procedures in the country. The Vanderbilt team published evidence that the surgery was beneficial in the Journal of the American Medical Association in 1999.
Paralysis, disabilities
Despite nationwide fortification of folic acid, the incidence of myelomeningocele has stabilized to 3.4 per 10,000 births. The layers of tissue and bone that normally cover and protect the spinal cord fail to close during development, leaving delicate nerves exposed to the intrauterine environment.
Children are often left with severe disabilities including paralysis below the waist, and lifelong bladder and bowel problems. Nearly 90 percent of children with this disorder develop hydrocephalus, a fluid build-up within the brain, which requires surgical placement of a shunt to drain fluid. A shunt, while necessary to save a child’s life, can impact intellectual development.
Landmark trial
The MOMS trial spanned seven years and included 183 participants. Half of the patients were randomized to receive fetal surgery to close their babies’ spines between the 19th and 25th week
of gestation.
The study was designed to address the “two-hit” theory, that in addition to the damage sustained during development, the nervous system continues to experience damage from environmental exposure in utero.
The MOMS trial found fetal surgery reduced the need for a shunt by almost 30 percent and significantly improved the child’s chances of being able to walk. There was no increased risk of death for the baby or the mother when the fetal surgery group was compared with a group that received surgery after birth.
Meet Emily
Before MOMS began, Vanderbilt had performed 177 fetal repairs. Maryann Dotegowski was patient No. 19.
“When they found the spina bifida, our specialist here in New Jersey said if I were his wife, he’d send me to Vanderbilt. We got on a plane as soon as we could,” Dotegowski recalls.
Today, her daughter, Emily, is an energetic middle-schooler who loves to sled. The family has returned to Nashville every year to see Tulipan for surgeries and follow-ups. Dotegowski says while Emily has significant physical challenges, she believes her daughter is able to walk because of the fetal surgery.
“Almost every doctor we talked to said she should be paralyzed from chest down,” Dotegowski said.
Overcoming doubts
Although spina bifida is disabling, it is not a lethal disease, and some critics said the risks were too great.
“There were a lot of people who were very much against it because it was possible the intrauterine surgery might kill the baby, or even the mother. We needed a randomized controlled study,” said Tulipan.
William Walsh, professor of pediatrics and director of nurseries at Vanderbilt, was one of the authors of the 1999 JAMA study, yet he recalls strongly advising parents of the known risks to their babies.
“It is so innovative to open the womb and operate at the limits of viability with the hope of improving a non-fatal illness. This is the first surgery of its kind and we knew there was a 100 percent chance of prematurity, so we had to be absolutely certain that benefits were greater than the risks,” said Walsh.
A family affair
When the trial began in 2003, Vanderbilt’s fetal surgery repair rate dropped from almost two a week to less than one a month. Families who participated were required to spend the remainder of pregnancy living close to the study center. Once the baby was born and discharged from the hospital, the families returned for 12- and 30-month follow-up assessments.
Even though participants’ expenses were covered, it was daunting.
“It was a tremendous burden to the team that was doing this procedure, because they felt it helped
children. But proving it was going to help it become the standard of care, otherwise the public, and the surgical communities might never have accepted this procedure,” Brock said.
Women’s health nurse practitioner, Mary Dabrowiak, one of the authors of the NEJM study and MOMS Coordinator, enrolled all 52 participants here at Vanderbilt.
“The families are the true heroes of this study,” she said. “Each one said to us that even if they
ultimately learned the procedure they had was not the most beneficial, they knew they were making the best choice, to help find the answers. Seven years later, the MOMS families continue their commitment, and the study sites have become a benchmark for spina bifida care,” Dabrowiak said.
Changing the conversation
MOMS showed there is risk inherent in the fetal surgery. Babies were generally born at 34 weeks of
gestation, often requiring a stay in a neonatal intensive care unit for breathing problems linked with prematurity. Mothers who have the procedure must deliver subsequent babies by Caesarian section because of a risk of uterine rupture.
“Because mothers are willing to go to extraordinary lengths to help their babies, and bear significant risks, even to their own health, we needed compelling evidence that the procedure is beneficial and safe. It is very satisfying to me that, with this new evidence, we can change our dialogue with families and offer the information they need to be able to make an informed decision,” said Kelly Bennett, the maternal/fetal specialist who performs the maternal portion of the surgery at Vanderbilt.
The study also shows further benefits for the baby, including a significant (30 percent) reduction of hindbrain herniation (Chiari malformation) in the patients who have undergone the fetal surgery – something Vanderbilt researchers also saw in the 1999 study. Tulipan says he is thrilled his previous findings have been reinforced, especially the reduction in the need for shunts.
“Shunts are what bring these patients back to the hospital over and over again. When a shunt fails it is a life-threatening situation. That’s something these patients walk around with hanging over their head all their lives. It is a huge benefit,” Tulipan said.
The study’s participants will continue to be followed. Several more questions remain to be answered. The children delivered during the MOMS trial, some of whom are as old as 7, will continue to be assessed to see if side effects of myelomeningocele like bladder and bowel control, and intellectual/learning disorders are impacted by fetal surgery.
The phone number for information about fetal surgery to repair myelomeningocele at Vanderbilt is (877) 875-3737 or in the Nashville area (615) 875-4500.
Visit the Vanderbilt website for information on fetal surgery, including the spina bifida repair.