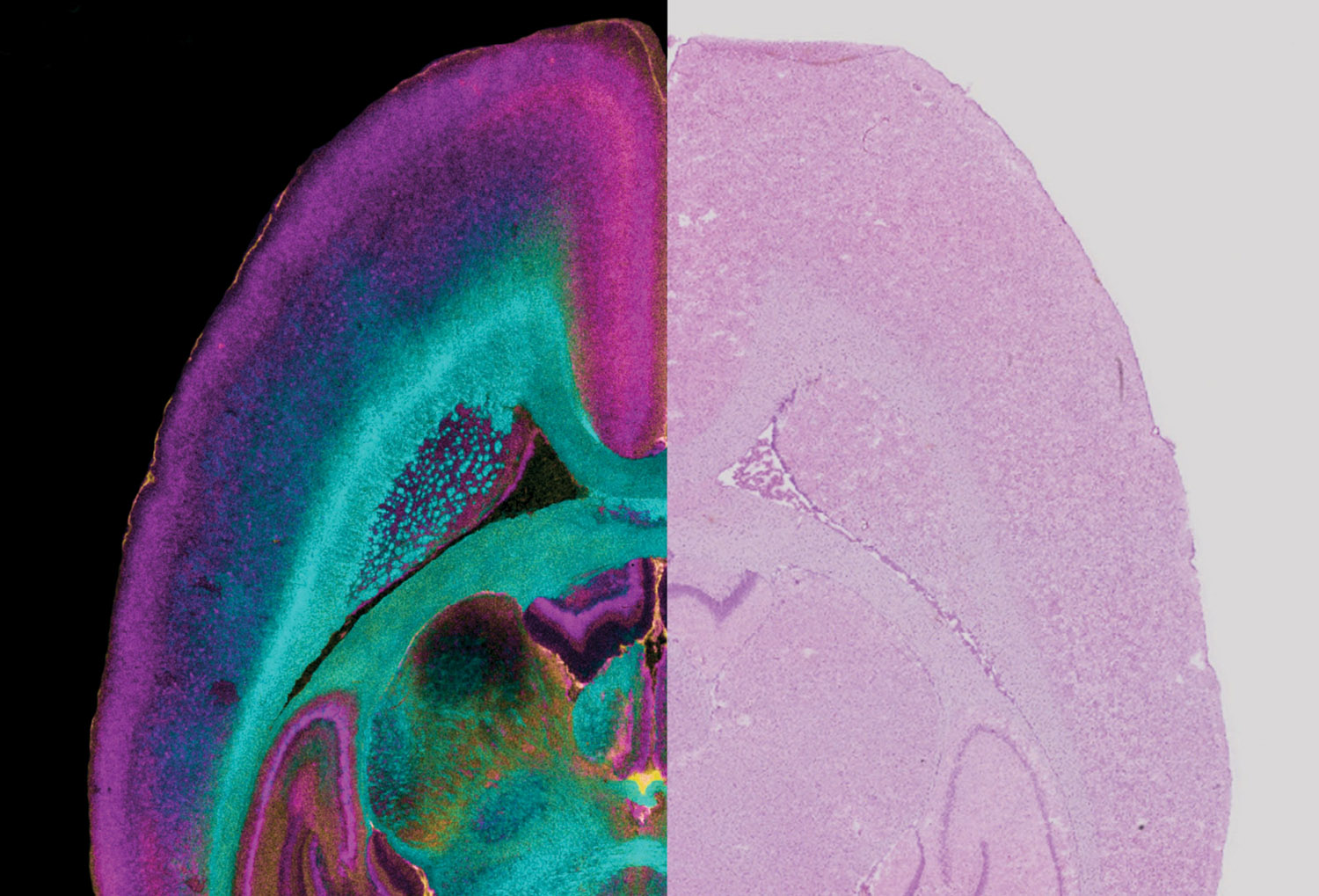
Recent advances in imaging technology are enabling Vanderbilt scientists to gain unprecedented views of how molecules, cells and tissues work together, yielding radical new insights into the causes, treatment and prevention of disease.
Across campus, faculty are applying new methods and technologies to generate detailed pictures of cellular mechanics. “Seeing leads to understanding,” says Lawrence Marnett, the School of Medicine’s dean of basic sciences and Mary Geddes Stahlman Professor of Cancer Research.
Thanks to these advances, “Vanderbilt scientists can determine the three-dimensional structures of molecular machines then watch them operate in intact cells, tissues or people,” Marnett says. “This helps us understand how they function in normal cellular physiology and what goes wrong in disease.
“Each of our imaging technologies operates at the state of the art,” he adds. “They have been implemented by Vanderbilt scientists who are defining the frontiers of their fields. The breadth and depth of our capabilities are awesome.”
PRECISION MEDICINE
The development of Vanderbilt’s imaging infrastructure owes much to Trans-Institutional Programs (TIPs), a series of university grants that supports collaborative research and infrastructure development from departments across campus. The $50 million annual TIPs initiative was launched in 2014.
Researchers in Vanderbilt’s Center for Molecular Probes and the Department of Chemistry, for example, are developing new radiopharmaceuticals—tracers—used to improve detection of cancer and other diseases. “Our efforts literally bridge molecules to man,” says center director Charles Manning, professor of radiology and radiological sciences in the School of Medicine.
As part of that work, Manning and his colleagues are attaching an imaging isotope to an experimental cancer drug and then using positron emission tomography (PET) to see where it goes. The goal is precision medicine—matching patients with the right therapy and monitoring outcomes with advances in imaging. “If used appropriately,” Manning says, PET “can reduce the cost of health care because we can make better decisions about the diagnosis and the treatment the patient should get.”
MACHINES OF LIFE
A second revolution brewing at Vanderbilt is the application and refinement of cryo-electron microscopy. For decades, X-ray crystallography has been the gold standard for visualizing the structures of proteins at the atomic level. But it’s not perfect. Some molecules and complexes will not crystallize, and the crystalline environment sometimes induces artifacts, or accidental features that interfere with the observation.
In the past few years, cryo-EM solved these problems and set off a revolution in the field. Single molecules and molecular complexes can be directly observed without any need for crystallization.
That is important, says Walter Chazin, Chancellor’s Professor of Medicine and director of the Center for Structural Biology, because it is these protein complexes—what he calls “machines of life”—that are key to understanding and developing more effective treatments for diseases like cancer.
“What’s happened in cryo-EM in the last five years has just been a stunning development in science,” adds Charles Sanders, the Aileen M. Lange and Annie Mary Lyle Professor of Cardiovascular Research and associate dean for research at the School of Medicine. “And we’re just getting started on this.”
Already the next-generation cryo-EM is on the market, and Vanderbilt is in line to get one, thanks to a TIPs grant awarded in 2017. Called the Titan Krios, the instrument sells for more than $6 million. It will be delivered to Vanderbilt in the fall.
MOLECULAR MAPPING
A third imaging revolution at Vanderbilt involves super-resolution light microscopy. Before 2011, light microscopes used by Vanderbilt scientists were limited to a resolution of about 200 nanometers. That is not sharp enough to pick up individual molecules within cells, which might be only a few nanometers wide.
Thanks to a computational advance called STORM (Stochastic Optical Reconstruction Microscopy), scientists now can calculate the size and location of molecules in cells and tissues.
“You can build maps of where all the molecules are,” says Matthew Tyska, the Cornelius Vanderbilt Professor of Cell and Developmental Biology and scientific director of the Cell Imaging Shared Resource.
STORM and another technique called SIM (Structured Illumination) are offered through the Nikon Center of Excellence for live-cell imaging at Vanderbilt, one of six in the country. The center opened in 2016 with financial support from the medical school’s Department of Cell and Developmental Biology and the Office of the Dean of Basic Sciences and with technical support from Nikon.
Another TIPs grant, awarded in 2017, is supporting efforts to build a new type of microscope that is not yet commercially available. Researchers in the School of Medicine, School of Engineering and College of Arts and Science are working on this new technology, called lattice light-sheet microscopy. It will enable scientists to perform long-term, high-resolution imaging over time, down to the molecular scale. Tyska says it is like having minutes-long “movies of life.”
Anita Mahadevan-Jansen, the Orrin H. Ingram Professor of Biomedical Engineering, is the grant’s principal investigator. Tyska and Shane Hutson, professor of physics and biological sciences and chair of the Department of Physics and Astronomy in the College of Arts and Science, are co-principal investigators.
DISEASE SIGNATURES
Despite recent progress, clinical imaging techniques—including magnetic resonance imaging—cannot see beyond the millimeter level. But it is at the micron-to-molecular scale where disease occurs. That is where a fourth research revolution at Vanderbilt comes in: imaging mass spectrometry and image “fusion.”
IMS, developed at Vanderbilt in the late 1990s, is essentially a molecular microscope that can measure the distribution, spatial rearrangement and alteration in expression levels of proteins, lipids and other biological molecules.
In 2015 a team led by Richard Caprioli, the Stanford Moore Professor of Biochemistry and director of the Mass Spectrometry Research Center, reported the first “image fusion” of mass spectrometry and microscopy—a major advance that allows scientists to see the molecular makeup of tissues in bright-field microscopic resolution.
“We’re now coming up with [molecular] signatures for disease,” Caprioli says. The technique can identify cells that look normal under the microscope but that already are transforming into cancer.
Caprioli predicts image fusion will have a huge impact on pathology and surgery. In removing a kidney tumor, for example, the imaging technology will give physicians a better idea of how much tissue should be removed to minimize the chance for recurrence.
Dr. Timothy Cover, professor of medicine and of pathology, microbiology and immunology, who is studying bacterial toxins, is looking forward to adding image fusion to his array of research tools.
“I think all these things will be complementary,” Cover says. “One of the things Vanderbilt is doing very well is staying competitive in multiple imaging areas.”
—WILLIAM SNYDER
A version of this story appeared in the Winter 2018 edition of Vanderbilt Medicine magazine.