Relieved undergraduates turned in their finals and jetted off for winter break a few weeks ago, leaving the halls at Vanderbilt University School of Engineering almost unnervingly empty.
But there’s no break for research. Anyone in need of company could find labs full of Ph.D. candidates working practically the whole time.
“We joke that one of the nice things about working during winter break is that it’s so quiet, you can get a lot of stuff done,” said Kelsey Beavers, a Ph.D. candidate in interdisciplinary materials science. “All of the instruments you need are available. All of the elevators are open exactly when you need them. You don’t have to avoid the class rush.”
While some students used the break to hit the slopes or holiday sales, here’s what a few of our researchers were doing.
Kelsey Beavers
Beavers describes microRNA as a “master puppeteer of gene networks” – a regulatory molecule that, if overactive, can be devastating for patients.
She’s working with Craig Duvall, assistant professor of biomedical engineering, developing nanotechnology that could deliver drugs for a broad range of applications, including regulating overactive microRNA so tissue can regenerate.
That means faster healing of foot wounds in diabetic patients. Skin healing for burn patients. Bone, kidney and liver regeneration.

“That’s why the engineering of the drug carrier is so important,” Beavers said. “We want to be sure we’re only regulating microRNAs in the diseased tissue. By controlling the makeup of the nanoparticle that delivers the drug, we can time our delivery, too. Maybe we only want to knock out the microRNA for a certain length of time.”
Beavers, who received her undergraduate degree from Georgia Tech, just finished her qualifying exam and has approval from her Ph.D. committee to move forward.
“It gives you momentum. You know that you have a specific plan of action. It’s exciting to just go full throttle and begin to knock out a lot of these experiments you’ve designed,” she said.
Sonia Brady
In the effort to find sustainable fuels, there’s a specific, significant bottleneck.
Sonia Brady, a Ph.D. candidate in chemical engineering, wants to widen it.
She studies how enzymes break down cellulose – research that could have significant applications in biofuels and help end the nation’s fossil fuel dependency.

It’s likely more practical to make biofuels from something other than corn, Brady said, which already has plenty of uses. Other biofuel feedstocks could be grown more quickly and pull fewer nutrients from the soil, and cellulose is the most abundant organic polymer on the planet.
“Corn is an efficient biofuel because they’re using the starch, not the cellulose in the cell wall of the plant,” said Brady, a Texas native. “If you looked at the corn husk, or grasses or trees that they’re looking at to use as biofuels, those are all cellulosic feedstock. It’s not high in starch content.”
Specifically, she’s looking at how cellobiohydrolases work. They are the enzymes that break down leaves on the forest floor.
“It’s a big issue. Our part is like this,” she said, holding her hands almost together.
She works in the lab of Matthew Lang, professor of chemical and biomolecular engineering.
Thomas Werfel
When cancer patients talk about getting a “dye” to find signs of their disease, they mean an imaging agent such as Fluorocoxib A. It’s used to detect COX-2, an enzyme that’s a reliable biomarker for many cancers.
The problem is that Fluorocoxib A doesn’t easily dissolve in water – which means it can’t have optimal performance in the body, and the kidneys clear it out quickly.
Thomas Werfel, a Ph.D. candidate in biomedical engineering from Waverly, Tenn., wants to take Fluorocoxib A to the molecular level. He’s working with tiny vials of purple solution that could hold the key to better cancer detection. The solution contains a new nanoparticle developed in the Duvall Lab that helps make Fluorocoxib A water-soluable, improving its delivery in the body.

Werfel said he and Craig Duvall, assistant professor of biomedical engineering, developed the idea after hearing a University of California-Los Angeles researcher discuss the problem. They started collaborating with Larry Marnett, associate vice chancellor of research and a cancer researcher in the Vanderbilt University Medical Center, and Md. Jashim Uddin, research assistant professor of biochemistry, two of Fluorocoxib A’s inventors.
“We all thought, ‘We could do that,’” said Werfel, who has a personal interest in the research because his mother is a breast cancer survivor. “All we needed was to find a way to make the molecule translatable to the clinic.”
Both Werfel and Beavers won National Science Foundation Graduate Research Fellowships for their work.
Electa Baker and Caroline Harriott
Most smartphone users are familiar with Google Maps – a layered system that can be as simple as routing commuters from point A to point B or as complex as providing brief information about businesses along the way, depending on the activated filters.
But in a crisis, emergency responders need maps with automatic filters that instantly pick out information they need to know. Otherwise, lives could be lost.

Electa Baker, who double majored in computer science and psychology with a focus on cognition at Bowling Green State University in Ohio, is trying to make computers think more like we do.
“If you show everything on the map, it’s just too much, and the relevant information gets lost,” said Baker, a computer science Ph.D. candidate. “When you filter that information, you have to pick the correct information, which is hard for a computer to do and not miss something important.”
She compares it to the way human brains easily tell the difference between a Pomeranian dog and a Persian cat. For a computer, with today’s technology, that’s a very difficult task.
Baker, who won a NASA Space Technologies Research Fellowship, works with Julie A. Adams, associate professor of computer science and computer engineering.
She is also assisting fellow computer science Ph.D. candidate Caroline Harriott in research about human-robot interaction, which also could aid first responders.
Harriott is evaluating whether human workers could perform tasks such as hazardous materials sampling or bomb disposal better with a robot companion.
“Rather than using the robot as a tool – like using the Mars Rover to search around – you’re treating it as a partner and teammate,” she said. “In my evaluation, the robot is coaching the first responder through the steps. I’m seeing whether you as a participant — if you have a robot partner or a human partner — which partner you do better with, which you trust more, which you prefer.”
Early results show better performance with human partners under some measures, Harriott said, but much of that may be attributable to the robot’s problems with voice communications.
Think Siri on a bad day.

Gilbert Rodriguez
You’re at a big summer barbecue, wiping away sweat and staring at that huge pan of potato salad the caterer brought.
But how long has it been out? Is it safe?

Gilbert Rodriguez, a Ph.D. candidate in electrical engineering, is working on a compact optical biosensor that could be used to detect almost instantly small concentrations of dangerous molecules, telling the user whether food is safe or not. The device also could have applications in the military and medical sectors.
Rodriguez’s lab-on-a-chip consists of a large array of biosensors on a surface the size of a penny, capable of detecting antigens, antibodies and proteins. It could be used to detect markers for E. coli and other bacteria and viruses.
“We want to make these cheap, portable, easy to use and useful for testing in water and other environments,” he said.
Rodriguez, who is from El Paso, Texas, said he chose the project because of its large scale impact in safety and preventative diagnostics.
Kevin Miller
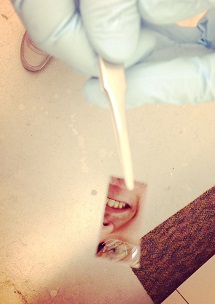
With a slight turn of the tweezers holding it, an inch-long, mirrored chip reflects the face of its creator.
Less obvious than Kevin Miller’s smile are tiny filters on the chip’s surface that he hopes will one day replace the electronic components inside computers.
Miller, a second-year student in the Interdisciplinary Materials Science Graduate Program, is developing a filter that can either capture or let through certain frequencies of light on demand. His optoelectronic device could replace current metallic components, allowing for potentially more energy-efficient computing.
“Working with light is the coolest thing in terms of science – at least, in my mind,” Miller said.
“It surrounds us in our everyday lives, so working in a field where I get to better understand and use it for a specific technology is exciting.”
The Jefferson City, Mo., native said he’s looking forward to a trip to Oak Ridge National Laboratory this month to use their equipment to fabricate his device.
Contact
Heidi Hall, (615) 322-6614
Heidi.Hall@Vanderbilt.edu
On Twitter @VUEngineering