There’s a new sheriff in town — in the world of microtubule dynamics.
Microtubules form the cell’s structural skeleton and cargo-moving system, and they are nearly always in flux. They grow, shrink and rearrange themselves as they participate in processes including cell division and migration.
Now, Vanderbilt University investigators have discovered a new molecular mechanism that regulates microtubule dynamics. The unexpected finding, reported in Developmental Cell, has implications for cancer drug discovery, said Irina Kaverina, Ph.D., associate professor of Cell and Developmental Biology.
“Cancer drugs that target microtubules in general have a lot of complications,” Kaverina said. “To find new, more selective drug targets, investigators are looking at the proteins that modify microtubule dynamics during cell division and cell migration.”
Microtubules are long tube-like structures that grow and shrink only at one end — the “plus end.” Proteins that bind at microtubule plus ends participate in the growing and shrinking processes. The end-binding proteins (EBs) — one of many types of proteins that bind the plus end — have claimed the crown as “master regulators of microtubule plus ends,” Kaverina said.
“The dogma is that EBs bind to microtubule plus ends and then every other protein that is there, it is there because it binds to EBs,” she said. “No one has considered whether one of these proteins regulates binding of EBs to microtubules.”
Kaverina’s group, led by former graduate student Ashley Grimaldi, Ph.D., found that a family of proteins called CLASPs, known to bind to the microtubule plus end, controls the binding of EBs to microtubules.
“Our paper is the first to show that another plus end protein regulates EBs,” Kaverina said.
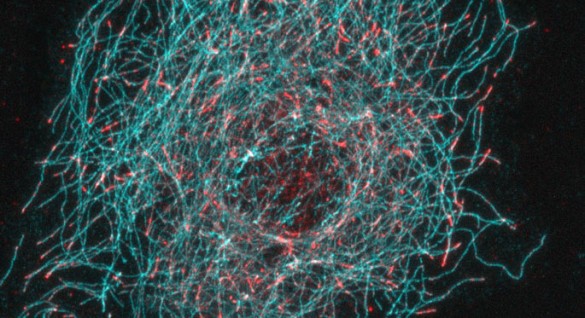
The researchers demonstrated that in cells without CLASPs, EBs were no longer concentrated at the dynamic plus end tip. Instead, EBs bound along the entire length of the microtubule.
In a series of biochemical experiments, they showed that CLASPs exclude EBs from binding along the microtubule length, restricting their localization to the microtubule plus end. The investigators suggest that CLASP alters the microtubule lattice to exclude EB binding, except at the tip.
“[lquote]Our findings change the whole concept of how microtubule plus ends are dynamically regulated,” Kaverina said.[/lquote]
Since EB proteins are targets of current cancer drug development programs, it’s especially important to consider the whole picture of their role in microtubule dynamics, she said. It might be possible to target CLASPs to influence EBs and microtubule dynamics.
Microtubules are targets not only for cancer drugs, but also for neurological diseases. In neurons, microtubules form the axon “tracks,” along which molecules are transported from the cell body to the nerve endings.
Kaverina emphasizes that the centrality of microtubules to cellular functions may make them good targets for the treatment of other disorders as well.
“I think there are very many understudied clinically important microtubule functions,” she said.
The research was conducted by a collaborative team including Kaverina’s group and investigators at Yokohama City University in Yokohama, Japan and at the University of Warwick in Coventry, United Kingdom. The work was supported in part by the National Institutes of Health (grant GM078373) and by the American Heart Association.