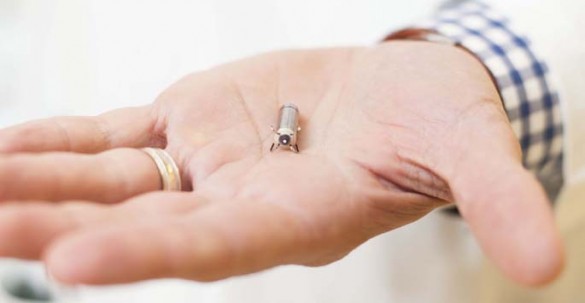
Vanderbilt Heart and Vascular Institute is participating in a global clinical trial to test the safety and efficacy of the world’s smallest pacemaker.
“This miniaturized technology is designed to provide patients with the advanced pacing technology of traditional pacemakers via a minimally invasive approach,” said Christopher Ellis, M.D., director of clinical arrhythmia research, who implanted the investigational device in a 59-year-old patient from Johnson City, Tennessee, on Sept. 9. Walter Clair, M.D., assisted Ellis.
Vanderbilt is the only hospital in Tennessee currently testing the Medtronic Micra Transcatheter Pacing System (TPS) and is one of 50 sites chosen to participate worldwide in the trial.
Pacemakers, the most common way to treat a slow or irregular heart rhythm, help restore the heart’s normal rhythm and relieve symptoms by sending electrical impulses to the heart to increase the heart rate.
The Micra TPS is one-tenth the size of a conventional pacemaker, and comparable in size to a large vitamin. It is best suited for patients needing a single chamber ventricular pacemaker. The small device is delivered directly into the heart through a catheter inserted in the femoral vein in the groin (trans-catheter delivery).

Once positioned, the pacemaker is securely attached to the heart wall and can be repositioned or retrieved if needed. The device does not require the use of wires (leads) to connect to the heart as traditional, larger pacemakers do. Attached to the heart via small tines, the pacemaker delivers electrical impulses that pace the heart through an electrode at the end of the device.
In contrast to current pacemaker implant procedures, the Micra TPS implant does not require a surgical incision in the chest and the creation of a “pocket” under the skin. It does not require a needle stick near the lungs either.
This eliminates two potential sources of complications, and any visible sign of the device.
“We are proud that Vanderbilt Heart was selected among an elite group of institutions to take part in this clinical trial. If positive, the results of the trial could potentially benefit the more than 1 million people globally who receive pacemakers each year,” Ellis said.
The Medtronic Micra TPS is an investigational device and has not yet received FDA approval.












