Mosquitos are involuntary bioterrorists.
For many years scientists thought that mosquitoes provided the disease organisms which they spread with a relatively free ride because the insects didn’t have much in the way of natural defenses to fight off these microscopic stowaways.
Recent research, however, has revealed that mosquitoes have surprisingly sophisticated immune systems. Unlike humans and most other animals, mosquitoes do not generate antibodies that identify and attack specific infectious agents. However, they have developed alternative methods for destroying various pathogens, including parasites that cause malaria.

In the latest study of the mosquito’s immune system – published in the November issue of the journal PLOS Pathogens – a pair of Vanderbilt biologists have discovered mosquitoes possess a previously unknown mechanism for destroying pathogens that takes advantage of the peculiarities of the insect’s circulatory system to increase its effectiveness.
Studies of this sort are providing the information needed to manipulate the mosquito immune system to block malaria parasites more effectively and to develop other novel disease control strategies.
“It may come as a surprise to many people, but mosquitoes get sick too and they need to protect themselves,” said Julián Hillyer, assistant professor of biological sciences, who conducted the research with graduate student Jonas King.
“The mosquito’s immune system isn’t as complex as ours. About 350 of its 12,500 genes have immune functions,” Hillyer said. “But it is remarkably effective. The vast majority of the malaria parasites that infect a mosquito die before they can get into the salivary glands where they can infect vertebrate prey, such as humans.”
“Pathogens like those that cause malaria, dengue and yellow fevers come from the female’s blood meal and end up in the mosquito’s gut,” Hillyer said. “They then leave the gut and enter the mosquito’s main body cavity, and from there they have to make their way to its salivary glands.”
Inside the body cavity, pathogens have to fight two main forces: the swift circulation of the mosquito’s own blood, and attacks from the mosquito’s immune system.
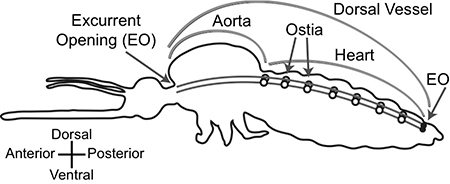
The mosquito’s circulatory system is dramatically different from that of mammals and humans. A long tube extends from the insect’s head to tail and is hung just under the cuticle shell that forms the mosquito’s back. The heart makes up the rear two-thirds of the tube and consists of a series of valves within the tube and helical coils of muscle that surround the tube. These muscles cause the tube to expand and contract, producing a worm-like peristaltic pumping action.
Most of the time, the heart pumps the mosquito’s blood—a clear liquid called hemolymph—toward the mosquito’s head, but occasionally it reverses direction. The mosquito doesn’t have arteries and veins like mammals. Instead, the blood flows from the heart into the abdominal cavity and eventually cycles back through the heart. “The mosquito’s heart works something like the pump in a garden fountain,” Hillyer said.
In order to make it to their goal, pathogens must pass through one of the heart valves. As a result, the valves act as physical bottlenecks during the migration of viruses and malaria parasites.
Cells called hemocytes are an important element in the mosquito’s defense system. These are special immune cells carried in the hemolyph that play a role analogous to white blood cells in humans. They circulate around the body with the hemolyph and attack foreign cells and viruses when they contact them.
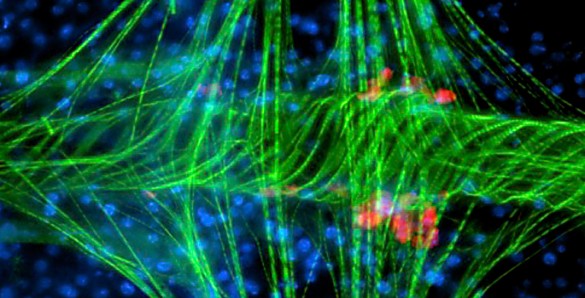
What Hillyer and King discovered was that when a mosquito becomes infected with bacteria or malaria parasites, a population of hemocytes are recruited to the valves of the heart, where they capture and destroy invading pathogens. These new mosquito immune cells, which they have named periostial hemocytes, substantially increase their odds of encountering and destroying invaders by congregating in areas of high hemolyph flow.
“What happens to these pathogens while they are carried inside the mosquito’s body is a critical part of the infection cycle that we are just beginning to understand,” said Hillyer.
This research was funded by the National Science Foundation grant number IOS-1051636.