Vanderbilt University researchers have good reason to feel proud about this year’s Nobel Prize in Chemistry, awarded last week to scientists at Duke and Stanford universities.
Several of them have collaborated with the Nobel laureates, Robert Lefkowitz, M.D., and Brian Kobilka, M.D., in their award-winning studies of G protein-coupled receptors (GPCRs) and the critical role that this diverse family of membrane proteins plays in cell signaling and disease.
“Bob was instrumental in inspiring my interest in pursuing biomedical research,” said Lee Limbird, Ph.D., Lefkowitz’s first postdoctoral fellow at Duke who went on to make major contributions to the GPCR field and to research at Vanderbilt. “His joy for discovery was palpable and contagious!”
During her six years in Lefkowitz’s lab in the 1970s, Limbird focused on beta-adrenergic receptors, GPCRs through which the catecholamines (epinephrine, norepinephrine and dopamine) exert their effects on heart rate, blood pressure and the body’s “fight-or-flight” response.
About half of all drugs target GPCRs. The beta-blocker drug propranolol, for example, lowers blood pressure by preventing adrenaline from binding to its GPCR.
After joining the Vanderbilt faculty in 1979, Limbird continued her research, even as she climbed the administrative ladder. She chaired the Department of Pharmacology throughout much of the 1990s, and was Vanderbilt’s first associate vice chancellor for Research before retiring in 2004.

For several years her lab collaborated with Kobilka, who as a trainee under Lefkowitz helped achieve the first cloning of a beta-adrenergic receptor. Kobilka delivered a Vanderbilt Discovery Lecture in 2007 after chalking up another “first” — the three-dimensional crystalline structure of the beta-2 adrenergic receptor.
“Kobilka is a scientist’s scientist,” said Limbird, currently dean of the School of Natural Sciences, Mathematics and Business at Nashville’s Fisk University. “And because he is a truth seeker, (he) is someone who sustains your faith in science and the discovery process.”
“(His) body of work has been a true inspiration to several in my laboratory who are using his techniques in their own studies,” added Tina Iverson, Ph.D., associate professor of Pharmacology at Vanderbilt.
Vsevolod Gurevich, Ph.D., is another Vanderbilt scientist with strong connections to the Nobel laureates.

He trained under another former Lefkowitz postdoc, Jeffrey Benovic, Ph.D., at Thomas Jefferson University in Philadelphia, and he collaborates with Kobilka in studies of arrestins, proteins that interact with hundreds of different GPCRs.
“As Bob puts it,” said Gurevich, a professor of Pharmacology who joined the Vanderbilt faculty in 2001, “I am one of his scientific ‘grandchildren.’”
Vanderbilt scientists have been among the pioneers in revealing how hormones and neurotransmitters regulate cellular processes.
They include Charles “Rollo” Park, Ph.D., who first elucidated insulin’s mechanism of action to include accelerated uptake of glucose into cells, the late Earl Sutherland, M.D., Vanderbilt’s first Nobel laureate in Medicine, who founded the concept of second messengers and identified cyclic AMP as the initial example, and Nobel laureate Stanley Cohen, Ph.D., who discovered a novel pathway for growth factor signaling.
Others include former Pharmacology Chair Joel Hardman, Ph.D., whose studies revealed the roles of guanylate cyclase and the second messenger cGMP in cellular regulation; and John Exton, M.D., Ph.D., who revealed the molecular pathways linking hormones interacting with GPCR to the elevation of another second messenger, calcium.
Jackie Corbin, Ph.D., and Sharron Francis, Ph.D., contributed to the discovery of the blood pressure drug that became Viagra, and Elaine Sanders-Bush, Ph.D., is internationally known for her work on serotonin receptors.

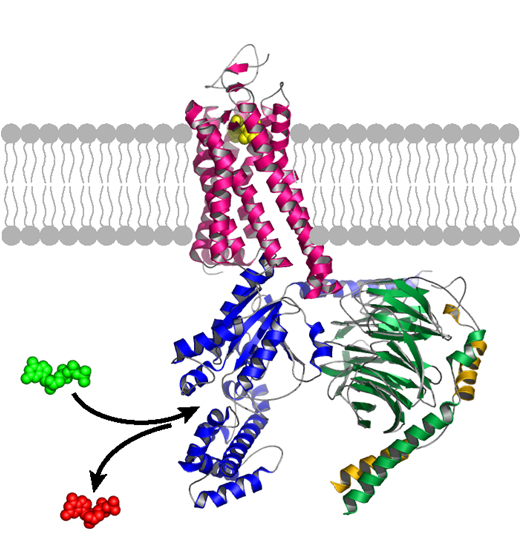
Current Pharmacology Chair Heidi Hamm, Ph.D., has made significant contributions to understanding the structure and function of the intracellular G protein, which binds to its particular GPCR when the receptor is activated, for example, by adrenaline.
Upon binding, the G protein splits apart, and each subunit can turn on a separate signaling pathway in the cell. The work of Hamm and her colleagues could lead to a new class of drugs, targeting the G proteins themselves.
“Many in the field have been trying intensely to unravel how receptors talk to G proteins,” said Hamm, who holds the endowed Aileen M. Lange and Annie Mary Lyle Chair in Cardiovascular Research.
Kobilka’s 3D structure, the first to show a G protein bound to its receptor, “is amazing to see,” she said. It “blows open the field to many new approaches such as structure-based drug design.”
P. Jeffrey Conn, Ph.D., who trained under Sanders-Bush and who directs the Vanderbilt Center for Neuroscience Drug Discovery, agreed.
The contributions by both Nobel laureates have been critical to the center’s efforts to find and help develop new drugs to treat Parkinson’s disease, schizophrenia and fragile X syndrome, the most common genetic cause of autism, said Conn, the Lee E. Limbird Professor of Pharmacology.
Others GPCR scientists at Vanderbilt include Richard Breyer, Ph.D., Roger Cone, Ph.D., Craig Lindsley, Ph.D., Terry Lybrand, Ph.D., and Ann Richmond, Ph.D.

Charles Sanders, Ph.D., professor of Biochemistry and a pioneer in the use of NMR techniques to determine the structure of membrane proteins, was particularly excited last week’s announcement.
Sanders witnessed Kobilka’s unveiling at a scientific meeting last year of the first 3-D structure of an active state GPCR in complex with its G protein. “When the rotating molecular image was projected onto the screen there was a hush of awe in the audience,” he said. “That was the most exciting moment of my scientific career.”















