A new technique for imaging whole cells in liquid – with a nanometer resolution that brings into focus individual proteins and other intracellular structures – could dramatically improve the study of cancer, viral infections and brain function.
The technique, electron microscopy (EM) of liquids, also may improve our understanding of reactions in energy storage materials, which has relevance for the development of improved batteries, and our understanding of liquid phase processes such as corrosion and electrochemical deposition.
Thus say two pioneers in the field: Niels de Jonge, assistant professor of Molecular Physiology and Biophysics at Vanderbilt University; and Frances Ross, a scientist at IBM’s T.J. Watson Research Center in Yorktown Heights, N.Y.
In a review article published this week in Nature Nanotechnology (de Jonge, N., and Ross, F.M., Electron microscopy of specimens in liquid, Nature Nanotechnology, 2011 Oct 23. doi: 10.1038/nnano.2011.161. [Epub ahead of print]), de Jonge and Ross describe a field that, as de Jonge puts it, “is about to become major.”
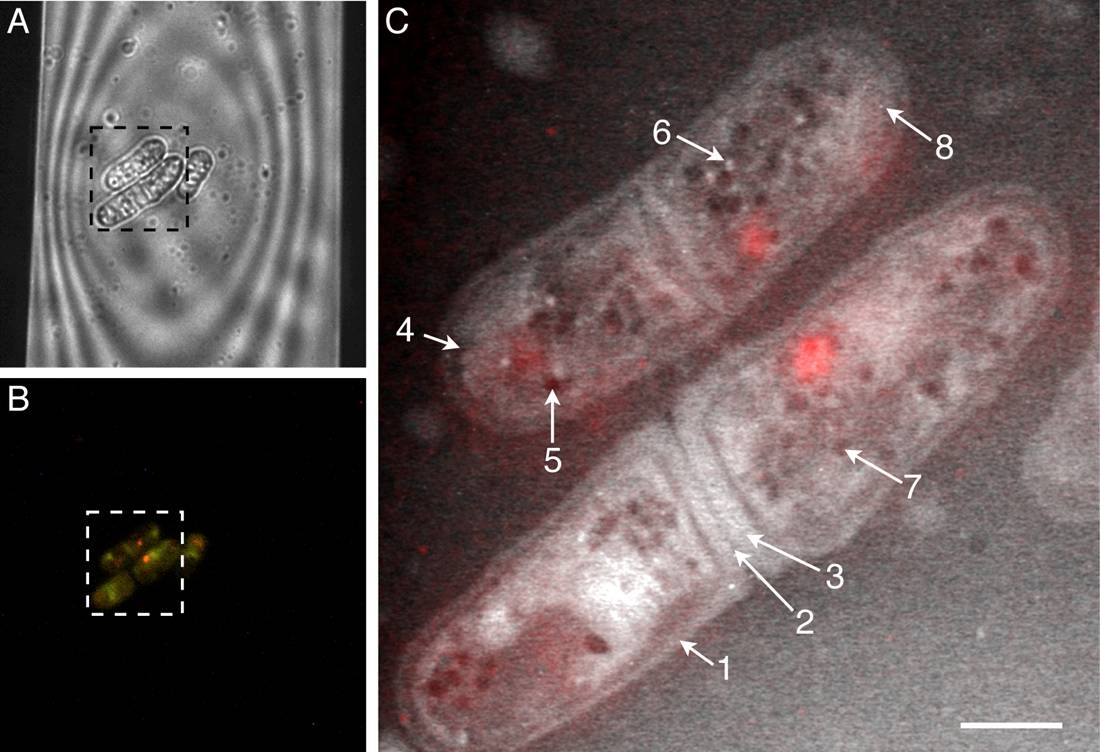
Caption: Light microscopy and liquid STEM of fully hydrated wild-type yeast cells. A. Cells are shown within a portion of the viewing window of the microfluidic chamber. B. Cells emit red fluorescence, indicating they are alive. C. Liquid STEM image of the same cells shows details at nanometer resolution, including cell wall (arrow 1), primary and secondary septa (dividing membranes; arrows 2 and 3), lipid droplet (arrow 5) and peroxisome (organelle; arrow 6).
Reprinted from Biophysical Journal, Vol. 100, Peckys DB, et. al., “Fully hydrated yeast cells imaged with electron microscopy,” pgs 2522-2529, May 18, 2011, with permission from Elsevier.
For decades, electron microscopy has been the gold standard for imaging molecules and other objects inside cells, many of which are smaller than the wavelength of light. But because regular EM requires a vacuum, samples must be fixed in a solid, non-living state.
In 2003, Ross and colleagues demonstrated how a liquid can be sealed inside a micro-fluidic device with electron transparent windows in the EM, allowing structures and processes to be observed in the liquid. In 2009, de Jonge and his colleagues at Vanderbilt and at Oak Ridge National Laboratory were the first to report the use of liquid EM to visualize labeled proteins in whole eukaryotic (complex) cells in liquid in exquisite detail, with a resolution of several nanometers.
Since then, de Jonge and his colleagues have used the technique to visualize the uptake of gold nanoparticles by living cells, image the intracellular “ultrastructure” of fully hydrated yeast cells, and study the locations of receptors specifically labeled with quantum dots.
Other researchers in Japan have used similar techniques to image the delicate synaptic connections between nerve cells and to study – at the cellular level — how cholesterol reduction suppresses tumor cell migration.
“We anticipate that electron microscopy of liquid samples will play an important role in solving a broad set of scientific problems,” de Jonge and Ross conclude.
And not just in biology and materials science. Liquid cell EM also may accelerate research on hydrated geological materials such as clays, they write, with implications for petroleum extraction and soil science.
Although electron microscopy in liquids has a history dating back to the earliest days of EM, recent technological developments have provided the required breakthroughs. The broad range of areas in which the technique could be applied suggests that numerous exciting developments can be expected in the future.