
The season in which babies are born can have a dramatic and persistent effect on how their biological clocks function.
That is the conclusion of a new study published online on Dec. 5 by the journal Nature Neuroscience. The experiment provides the first evidence for seasonal imprinting of biological clocks in mammals and was conducted by Professor of Biological Sciences Douglas McMahon, graduate student Chris Ciarleglio, post-doctoral fellow Karen Gamble and two undergraduate students at Vanderbilt University.
The imprinting effect, which was found in baby mice, may help explain the fact that people born in winter months have a higher risk of a number of neurological disorders including seasonal affective disorder (winter depression), bipolar depression and schizophrenia.
“Our biological clocks measure the day length and change our behavior according to the seasons. We were curious to see if light signals could shape the development of the biological clock,” said McMahon.
In the experiment, groups of mouse pups were raised from birth to weaning in artificial winter or summer light cycles. After they were weaned, they were maintained in either the same cycle or the opposite cycle for 28 days. Once they were mature, the mice were placed in constant darkness and their activity patterns were observed.
The winter-born mice showed a consistent slowing of their daily activity period, regardless of whether they had been maintained on a winter light cycle, or had been shifted to summer cycle after weaning. When the scientists examined the master biological clocks in the mouse brains, using a gene that makes the clock cells glow green when active, they found a similar pattern: slowing of the gene clocks in winter-born mice compared to those born on a summer light cycle.
[lquote]“What is particularly striking about our results is the fact that the imprinting affects both the animal’s behavior and the cycling of the neurons in the master biological clock in their brains[/lquote],” said Ciarleglio.
In addition, their experiments found that the imprinting of clock gene activity near birth had dramatic effects on the reaction of the biological clock to changes in season later in life. The biological clocks and behavior of summer-born mice remain stable and aligned with the time of dusk while that of the winter-born mice varied widely when they were placed in a summer light cycle.
“The mice raised in the winter cycle show an exaggerated response to a change in season that is strikingly similar to that of human patients suffering from seasonal affective disorder,” McMahon commented.
Exactly when the imprinting occurs during the three-week period leading up to weaning and whether the effect is temporary or permanent are questions the scientists intend to address in future experiments.

Seasonality and Personality
The new study raises an intriguing but highly speculative possibility: Seasonal variations in the day/night cycle that individuals experience as their brains are developing may affect their personality.
[rquote]“We know that the biological clock regulates mood in humans. If an imprinting mechanism similar to the one that we found in mice operates in humans, then it could not only have an effect on a number of behavioral disorders but also have a more general effect on personality[/rquote],” said McMahon.
“It’s important to emphasize that, even though this sounds a bit like astrology, it is not: it’s seasonal biology!” McMahon added.
Mice in this study were raised on artificial seasonal light cycles in the laboratory and the study was repeated at different times of the year. In humans, studies conducted in the northern and southern hemispheres have confirmed that it’s the season of winter – not the birth month – that leads to increased risk of schizophrenia. There are many possible seasonal signals that could affect brain development, including exposure to flu virus. This study shows that seasonal light cycles can affect the development of a specific brain function.
“We know from previous studies that light can affect the development of other parts of the brain, for example the visual system. Our work shows that this is also true for the biological clock,” said Ciarleglio.
Background
The experiment was performed with a special strain of genetically engineered mice that it took McMahon two years to develop. The mice have an extra gene inserted in their genome that produces a naturally fluorescent green protein causing the biological clock neurons in their brains to glow green when they are active. This allows the scientists to directly monitor the activity of the master biological clock, which is located in the middle of the brain behind the eyes in a small area called the suprachiasmatic nucleus (SCN).

For the study, the researchers took three groups of six to eight newborn pups each and placed them (and their mothers) in environments with controlled day/night cycles. One group was placed in a “summer” cycle with 16 hours of light and eight hours of dark; another group was placed in a “winter” cycle with eight hours of light and 16 hours of dark; and a third group was placed in an equinox cycle with 12 hours of light and 12 hours of darkness. They were kept in these environments for three weeks until they were weaned.
“When they are born, the brains of mice are less developed than those of a human baby. As a result, their brains are still being wired during this period,” McMahon said.
Once they were weaned, half of the summer-born mice were kept on the summer cycle and half were switched to the winter cycle for the following 28 days as they matured. The winter-born mice were given the same treatment. The equinox-born mice were split into three groups and put into summer, winter and equinox cycles.
After the mice matured, they were placed into an environment of continuous darkness. This eliminated the day/night cues that normally reset biological clocks and allowed the scientists to determine their biological clock’s intrinsic cycles.
The scientists found a substantial difference between the summer-born and winter-born groups.
The summer-born mice behaved the same whether they had been kept on the summer cycle or switched to the winter cycle. They started running at the time of dusk (as determined by their former day/night cycle), continued for ten hours and then rested for 14 hours.
The behavior of the winter-born mice was much different. Those who had been kept on the winter light cycle through maturation showed basically the same pattern as their summer cousins: They became active at the time of dusk and continued for 10 hours before resting. However, those who had been switched to a summer cycle remained active for an extra hour and a half.
When they looked at what was happening in the brains of the different groups, they found a strikingly similar pattern.
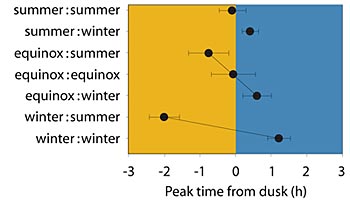
In the summer-born mice, the activity of the neurons in the SCN peaked at the time of dusk and continued for 10 hours. When the winter-born mice were matured in the winter cycle, their neuronal activity peaked one hour after the time of dusk and continued for 10 hours. But, in the winter-born mice switched to a summer cycle, the master bioclock’s activity peaked two hours before the time of dusk and continued for 12 hours.
When they looked at the equinox group, the scientists found variations that fell midway between the summer and winter groups. Those subjected to a summer cycle when they matured had biological clocks that peaked one hour before the time of dusk and the biological clocks of those subjected to a winter cycle peaked a half hour after the time of dusk. In both cases the duration of SCN activity was 11 hours.
Their analysis showed that these variations are caused by alterations in the activity patterns of the individual neurons, rather than by network-level effects.
[lquote]“It is quite striking how closely the neuronal wave form and period line up with their behavior[/lquote],” McMahon said.
Ciarleglio completed his graduate studies and is now assistant director of the Vanderbilt Brain Institute. The undergraduate contributors to the study were John Axley and Benjamin Strauss, who have graduated and gone onto graduate school and medical school. Karen Gamble, the contributing post-doctoral fellow, is now a faculty member in the psychiatry department at the University of Alabama Birmingham.
The research was funded by grants from the National Institutes of Health and was conducted in association with the Silvio O. Conte Neuroscience Research Center at Vanderbilt.