Since launching its program offering patients with non-valvular atrial fibrillation (AF) an alternative treatment to reduce their risk of stroke and avoiding long-term use of blood thinners, the Vanderbilt Heart and Vascular Institute (VHVI) has consistently ranked among the top centers in the country for the implantation of left atrial appendage closure (LAAC) devices.
Patients with AF, the most common form of arrhythmia, have an increased risk of stroke because blood can pool and form clots in an area of the heart called the left atrial appendage, or LAA. A clot can break free from the LAA and travel to the brain, causing a stroke.
For more than 50 years, oral blood thinners were the only intervention for reducing the incidence of stroke in AF patients.
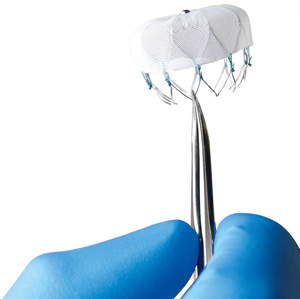
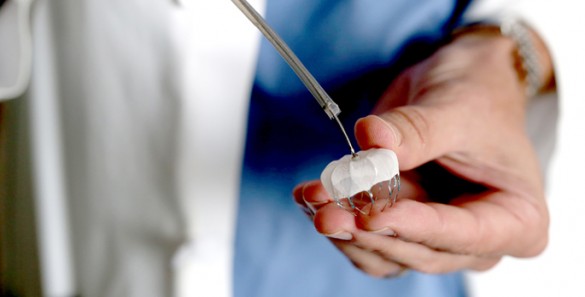
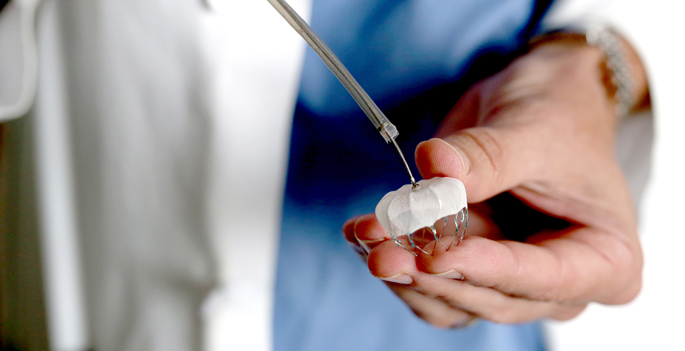
Currently, patients have three devices to choose from: the Watchman LAAC, the Amulet device as part of the AMPLATZER Amulet IDE trial, and the Lariat closure, which is a part of the aMAZE clinical trial.
VHVI is now the No. 1 enrolling site for the current AMPLATZER Amulet IDE trial, a head-to-head look at two LAAC devices. Vanderbilt leads enrollment in the trial and is one of 100 sites across the country participating in the randomized, controlled study.
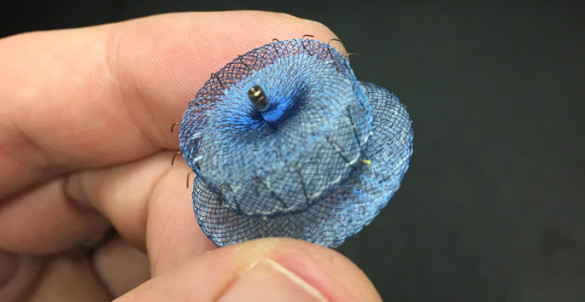
VHVI also recently implanted its 200th Watchman LAA occluder, an FDA-approved device that propelled the center into becoming the largest program in the state and Southeast for LAA closures for that device. It has a comprehensive program offering a variety of LAAC options for AF patients and expects to introduce an additional device (Watchman FLX) this summer.

“Regionally, we are probably the only site that can offer all of these options,” said Christopher Ellis, MD, associate professor of Medicine and director of Vanderbilt’s LAA Closure Program.
“It’s nice to see that over a three-year period our program is growing fast, with excellent outcomes and has potential for becoming the standard of care nationwide in AF related stroke prevention.
“Our goal is to be able to implant 99 percent or more of the patients who come into our facility in need of this intervention (LAA closure) with less than 1 percent complication rate. LAA closure is unique. After it is implanted, there is essentially 100 percent compliance with therapy.”
“Although effective, blood thinners carry a significant rate of bleeding complications, and we are finding that as many as one-third of patients stop taking their prescribed doses after one year because of adverse bleeding, fear of complications and cost of the medication.”
LAA closures are aimed at patients with AF who have an increased stroke or risk score and can be safely removed from the blood thinner protocol.
Ellis said not only have the outcomes been impressive, but data is showing that these devices are more cost effective than long-term medication use.
Despite the success with the new line of LAA targeted therapy, Ellis said there is still a huge need among the medical community to educate patients.
“There is a great need for awareness about this therapy and learning to accept that it is a safe and effective alternative to blood thinners,” said Ellis. “We are working on ways to identify patients as they come into the ED on a blood thinner with a diagnosis of intracranial hemorrhage or GI bleeding or other bleeding complications that require them to stop their blood thinner regime.”
Ellis, along with Robert Piana, MD, professor of Medicine and Arvindh Kanagasundram, MD, assistant professor of Medicine, implant the Watchman devices.