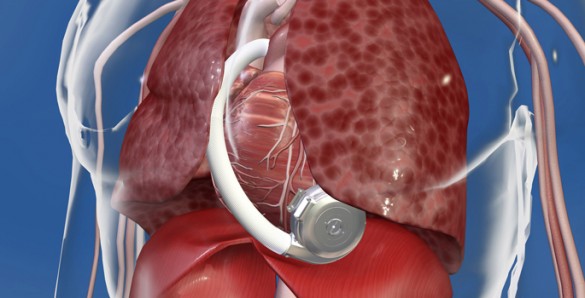
Vanderbilt Heart and Vascular Institute (VHVI) reached a significant milestone recently with the 100th implantation of the miniaturized HeartWare HVAD Pump, a left ventricular assist device (LVAD) that supports the heart.
On Oct. 3, physicians, nurses and other cardiac caregivers gathered to celebrate and acknowledge the success of the LVAD program.
“This is an important milestone as Vanderbilt has become the first center in North America to reach 100 HeartWare LVAD implantations since the device was FDA approved in 2012. Our institution has become the largest implantation center of the HeartWare device in the U.S.,” said Simon Maltais, M.D., assistant professor and director of the Heart Transplant and Ventricular Support Program.
The HVAD Pump is a compact, full output device that is implanted in the pericardial space in the heart. It sits inside the chest cavity and connects directly to the heart.
No abdominal dissection is required in the implant procedure. It can also be implanted through a minimally invasive approach known as a thoracotomy, a small incision on the side of the chest. This can avoid opening the chest through a sternotomy in some patients, thus decreasing OR time, risk of perioperative bleeding and infection.
“Left ventricular assist devices improve both survival and quality of life for many patients with end stage heart failure, enabling the majority of patients to reengage in work and other activities that were not previously possible,” said Mary Keebler, M.D., medical director of the Ventricular Assist Device Program.
In another milestone, Vanderbilt Heart recently performed its 400th transcatheter aortic heart valve-replacement (TAVR).
Vanderbilt performed its first TAVR in July 2011 when it embarked on the clinical trial of Medtronic’s CoreValve.
Vanderbilt is one of a few medical centers in the country to offer both the CoreValve and the Edwards SAPIEN valve, artificial valves designed to replace a patient’s defective one without opening the chest.
“To reach this milestone in such a short timeframe is a tremendous accomplishment. We have clearly established Vanderbilt as a leader in this minimally invasive procedure for valve replacement,” said Michael Petracek, M.D., chair of the Department of Cardiac Surgery.
The CoreValve is made from porcine pericardial tissue with an expandable frame. Using transcatheter aortic valve implantation, the artificial valve is attached to a wire frame and guided by catheter through the groin to the heart.
Compared to the four to six hours typical in open-heart surgeries, this procedure calls for a smaller incision and takes about 90 minutes to complete.