by Jessica Mazerik
In an article published in December in Proceedings of the National Academy of Sciences, researchers at Vanderbilt University, the University of Geneva Medical School and Avid Radiopharmaceuticals, Inc., described an imaging protocol to hunt for molecules that will light up beta cells, rare but vital insulin-secreting cells that make up less than one percent of the pancreas mass. “Imaging beta cell mass would be a great biomarker for the diabetes community,” said Jack Virostko, a postdoctoral fellow in the Vanderbilt University Institute of Imaging Science (VUIIS) and the paper’s lead author.
Beta cells reside in the pancreas and secrete insulin in response to spikes in blood sugar levels. In people with diabetes, the beta cells do not function properly, and are often reduced in number. Because beta cells make up such a small percentage of the pancreas, distinguishing them from surrounding tissue in imaging applications poses a difficult problem. The ability to image beta cells in a patient would help doctors diagnose diabetes earlier and monitor changes in beta cell mass in response to treatments.
The right molecule
“Physicians would love to have that kind of a measurement,” said Virostko, who headed up the imaging part of the study. Physicians think some therapies that result in improvements in diabetes patients actually may increase beta cell mass, but there is currently no way to test this idea. The screening technique designed by Virostko and colleagues means imaging beta cell mass is a real possibility once the right imaging molecule is found.
Designing a method to test molecules called tracers for the ability to bind specifically to beta cells was not an easy task. “In order to do these experiments, it required a lot of different people with a lot of different skill sets,” said Virostko.
Together with colleagues at VUIIS and the Vanderbilt Diabetes Research and Training Center, Virostko imaged mice using positron emission tomography (PET), which detected tissue through the use of radioactive tracers, and bioluminescence, which detected only beta cells genetically modified to express luciferase, the same protein that makes fireflies glow. Combining these two techniques, the authors pinpointed the beta cells within the abdominal cavity of the mouse.
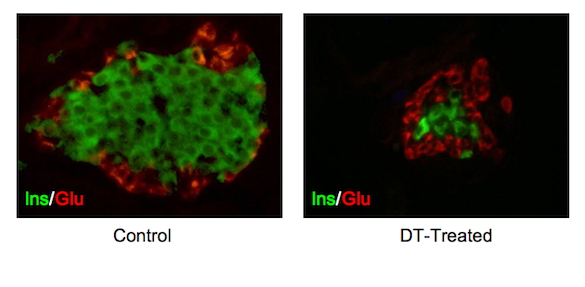
The Vanderbilt researchers next teamed with chemists at Avid Radiopharmaceuticals, which had designed PET tracers thought to only detect the beta cells. The Vanderbilt researchers combined a mouse model developed at Vanderbilt with one created at the University of Geneva Medical School. This new mouse model had beta cells that lit up through bioluminescence, and which also expressed the diphtheria toxin receptor. When the mice were injected with diphtheria toxin, only the beta cells expressing the receptor would be killed.
The mice were imaged with PET and bioluminescence before injection with diphtheria toxin. If the tracers were working properly, the PET and bioluminescence signals would light up only in the beta cells. After the toxin injection, no beta cells would be visible because they did not survive to bind the tracers.
Unexpected finding
To take the screening procedure a step further, the Vanderbilt researchers designed a preclinical model. They transplanted human beta cells into living mice and tested PET tracers for the ability to detect the human cells. The human tissue was provided by the national Integrated Islet Distribution Program, which is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health with support from Juvenile Diabetes Research Foundation.
Surprisingly, the PET tracers used in the study did not bind specifically to beta cells in mice or in transplanted human islets. Although this finding was unexpected, a useful tool was created to test PET tracers for the ability to specifically label small groups of cells. “Traditional radiology has dealt with big structural things,” Virostko said. “If you have a broken bone, that’s anatomically easy to detect. But if you’re just losing these small populations of cells, that’s not something you can detect using traditional radiology.”
“Now that we’ve worked out the protocol, we can test a compound very quickly, much more quickly than was possible before,” he said. The Vanderbilt group is now using this new model to search for specific beta cell imaging molecules.
“This type of interdisciplinary work can only be done at places like Vanderbilt,” Virostko added. The overall experimental approach is applicable to other cell types and different molecular labeling methods: the technique could also screen for molecules to detect cell populations affected by diseases like Parkinson’s or Alzheimer’s, he said.
Other Vanderbilt researchers who contributed to this study were Aramandla Radhika, senior research assistant in the Diabetes Center; Todd Peterson, assistant professor, radiology and radiological sciences; Joseph Henske, former clinical diabetes fellow; Chunhua Dai, research assistant professor of medicine; Ronald Baldwin, former research associate professor, radiology and radiological sciences; Mohammad Ansari, manager, PET Radiochemistry Core Lab, VUIIS; and Alvin Powers, Joe C. Davis Chair in Biomedical Science and director of the Vanderbilt Diabetes Center.
Their study was supported in part by the JDRFand several NIH grants, including 5U01DK072473, 5U01DK089572, 5R21DK068854, 5R33DK066636, 5R01DK068764, 3R01DK069603 and 5T32EB001628.